Table of Contents
It’s hard to reckon specifically when in record individuals grew to become a technological species. Aspect of that is for the reason that the definition of technological know-how is to some degree subjective if you think earning a stick pointy plenty of to grub roots from the dirt or to poke sufficient holes in an animal to convince it to permit you try to eat it is technology, then our engineered planet goes back a lengthy, very long way without a doubt.
But anything about pointy sticks just does not seem transformative more than enough, in the perception of basically transforming a by natural means happening substance, to truly depend as a technological line in the sand. To cross that line, it truly would seem like the use of metals need to be element of the package deal. Even if which is the case, our technological heritage still goes rather far back again. And copper finishes up being a person of the metals that began it all, about 11,000 yrs ago, when our ancestors found purely natural deposits of the smooth, reddish metallic and began discovering how to style it into the instruments and implements that lifted us out of the Stone Age.
Our environment virtually are not able to operate without copper, forming as it does not only the electrical-motor muscle groups of civilization, but also the wires and cables that form the ability and facts grids that stitch us collectively. Ironically, we are just as dependent on copper now as we have been when it was the only steel we could make applications from, and potentially a lot more so. We’ll choose a glimpse at what’s involved in extracting and purifying copper, and see how the solutions we now use are not solely diverse from these formulated over 7 millennia in the past.
Shiny Rocks
As helpful as copper was to early civilizations, and for as conveniently available as it was many thanks to surface deposits of native copper sprinkled about the world, it was not the very first metal to be uncovered and labored. That honor falls to equally gold and, strangely more than enough, meteoric iron. But neither of these metals was abundant adequate to make anything but a token influence on know-how, and generally ended up enriching and ornamenting kings and princes.
Copper, nevertheless, was easily located and, possibly more importantly, easily labored devoid of the need to produce a lot infrastructure — at the very least at 1st. Lumps of copper could be pried from native copper deposits and cold-labored with stone tools into useful artifacts, many thanks to copper’s malleability. It was not extensive right before copper’s comparatively low melting point led to the discovery of casting, which led to additional employs for the metal and amplified demand from customers.

Finally, supplies of indigenous metal from quickly exploited deposits exceeded need, and our ancestors found out smelting from many copper-bearing ores. The most significant ore for commercial copper creation is called chalcopyrite, an iron-containing copper sulfide mineral with the chemical components CuFeS2. Chalcopyrite deposits are found all more than the globe, with particular abundance in North and South The usa, as properly as Africa and Australia. Other vital ores happen as oxides and carbonates of copper, like azurite and cuprite.
Although some deep-shaft mining is finished, most of the big copper mining functions are extensive open up-pit mines. The world’s most successful copper mine appropriate now is the Minera Escondida in the Atacama Desert in Chile, which developed $10 billion value of copper in 2007 and can output 1.2 million tons a calendar year. Whilst a pure sample of chalcopyrite is about 34% copper by bodyweight, the mineral is normally affiliated with a host rock species that lowers the ore to a fraction of a p.c of copper. This means that broad amounts of ore have to be processed to make a mining operation commercially feasible. In some deposits, gold and silver are sparingly substituted for copper in the ore, creating these important metals a important aspect products that in some situations can actually fork out for the full cost of extraction of all the copper.
Extraction in open up-pit mines begins with regular hard-rock mining strategies, like blasting. Ore-bearing rock is loaded 200 to 300 tonnes at a time by tremendous loaders and shovels into mammoth haul trucks, for the trip up out of the pit to the processing plant. There, massive crushers decrease the car-sized boulders into lesser and lesser fractions, which are passed to ball mils for finer grinding. The objective is to decrease the actual physical make contact with involving the ore minerals and the squander rock that surrounds it, which is known as gangue.
What transpires future is the extraction of the elemental copper from the ore minerals, but the method applied is dependent upon which variety of ore is existing. For oxides and carbonates of copper, the copper is soluble in acid options, so a hydrometallurgical process is utilized. Particulars change, but in leaching procedures, usually the powdered ore is piled up in huge pits lined with an impervious barrier. Dilute sulfuric acid is sprayed on to the piles and leaches copper sulfate from the ore minerals. The copper is stripped from the leachate with unique extractants, which leaves the sulfuric acid clean up and all set to be recycled for a further round of leaching, plus a copper-loaded solution completely ready for more purification.
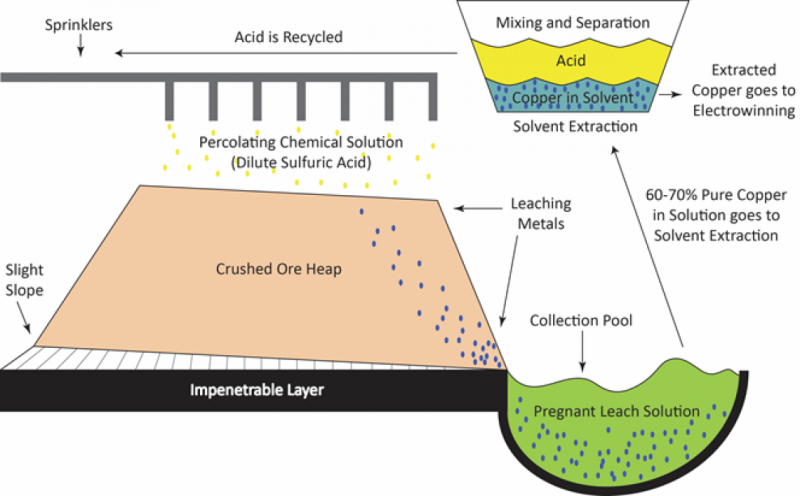
Floating to the Prime
Copper sulfide ores are at a downside when it arrives to chemical strategies of extraction, because the sulfides are scarcely soluble in acid. To free copper from these ores, refiners need to have to switch up the heat with pyrometallurgical approaches. These start off with the same crushing and grinding methods as just before, ensuing in a high-quality powder that is mixed with drinking water in huge vats. To the slurry are extra chemicals known as collectors, whose work it is to bind to the sulfide mineral particles. The collector molecules go over the sulfide particles and enhance their hydrophobicity, or tendency to repel drinking water, when leaving the squander rock particles by itself. When air is bubbled through the alternative, the now-hydrophobic sulfides connect to the air bubbles and form a froth at the floor of the vat, which is skimmed off the top rated and subjected to additional rounds of this froth flotation system to boost the concentration of copper.
The output from this froth flotation approach is then place by a thickening process, to take out as much drinking water as feasible. This is accomplished by a blend of simple evaporation in open up ponds, and by filtration employing porous ceramic discs or cylinders. The filtration move is crucial, as it lowers the humidity content to all-around 8% and benefits in a copper concentrate of about 20-30% enrichment that can be quickly shipped to smelting vegetation.
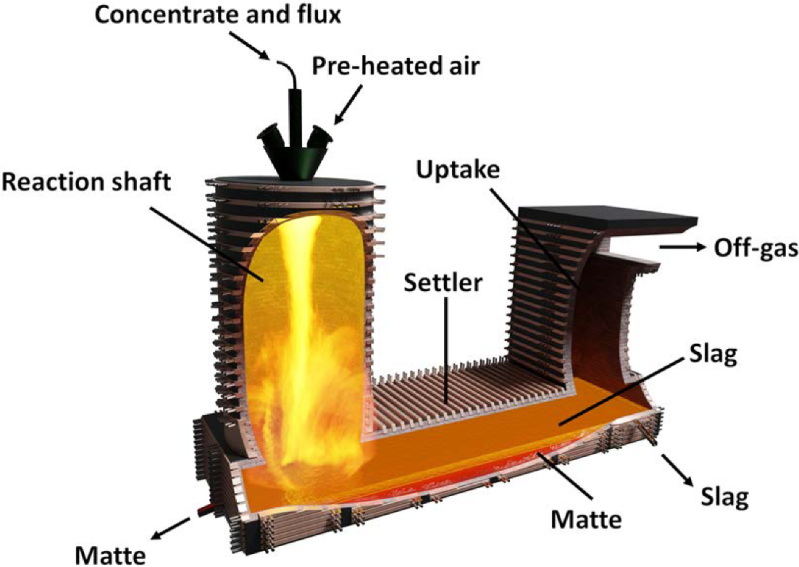
To extract the copper from the concentrate, a flash smelting method is used. Concentrate is sprayed into a reaction column in a flash furnace alongside with heated, oxygen-enriched air. The sulfide compounds in the focus melt immediately and tumble to a collecting pool at the base of the furnace. There, the molten components separate by density, with the molten copper, known as matte copper, sinking to the bottom, whilst the iron and silicate slag floats to the top with the aid of extra fluxes.
Matte copper, now about 60% pure, is tapped off the bottom of the flash furnace for further purification as a result of conversion, which is mainly blowing warm air by the molten matte. The oxygen reacts with the remaining sulfur, leaving at the rear of blister copper which is about 98% pure.
Winning with Electrical energy
The remaining stage of purification for the close solutions of both equally hydrometallurgical and pyrometallurgical extraction is identified as electrowinning. This is simply electrolysis, albeit on a significant scale. For hydrometallurgical copper, the copper sulfate solution that will come from the leaching pit is utilised as the electrolyte, with direct anodes and slim stainless metal sheets for cathodes. Existing is handed through the electrolyte, creating the copper in the option to plate out onto the stainless steel cathodes. When about 100 lbs . (45 kg) of copper have amassed on the cathodes, they are removed, rinsed, and flexed to pop off the completed, 99.99% pure copper sheets.
For pyrometallurgical copper, the blister copper ingots provide as anodes for electrowinning. They are suspended in a tank filled with dilute copper sulfate mixed with sulfuric acid, interleaved with cathodes of both pure copper sheets or, once more, stainless metal. Recent is handed by means of the tank and the copper plates out on the cathodes, once again achieving 99.99% purity in the finished procedure.
The waste product left behind in the electrowinning tanks is acknowledged as anode slime, and irrespective of its unappealing identify is a useful merchandise. Relying on the minerals present in the feedstock and the voltage employed for electrowinning, the anode slime can have gold, silver, selenium, tellurium, and maybe even platinum-group metals, along with a reasonable sum of copper that wasn’t recovered in the to start with go-all-around. Anode slime is frequently marketed off to specialty smelters for recovery of these precious metals, applying combinations of hydrometallurgical and pyrometallurgical procedures that are personalized to the blend of metals in the slime.
Banner impression: “Native copper-changed cross-bedded sedimentary rocks” by James St. John, CC BY 2.





